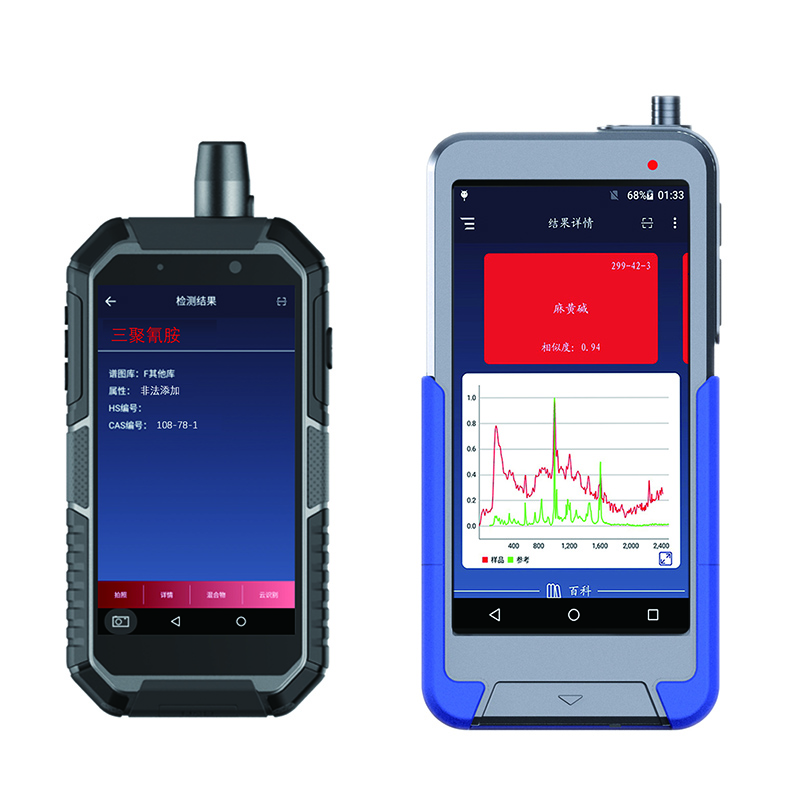
RS1000DI RS1500DI handheld Raman Identifier
★ Amplis deprehendendi, chemica, diam materiarum rudium et pigmentorum invenire possunt
Protinus explorari potest per vitreas, intertextas, saccos, chartas, materias, et alia packaging RS1500DI)
★ Parvum et leve, flexibiliter moveri potest in horreis, praeparationibus materialibus cubiculis, officinae productionibus et aliis locis.
Velox responsio et idem perfici potest in secundis
★ Non opus est sampling, non opus est materias crudas et auxiliares ad sampling cameram transferre, quae sampling contaminationem vitare potest.
Accurate identificatio, utens machina eruditionis algorithmus, fortis specificitas
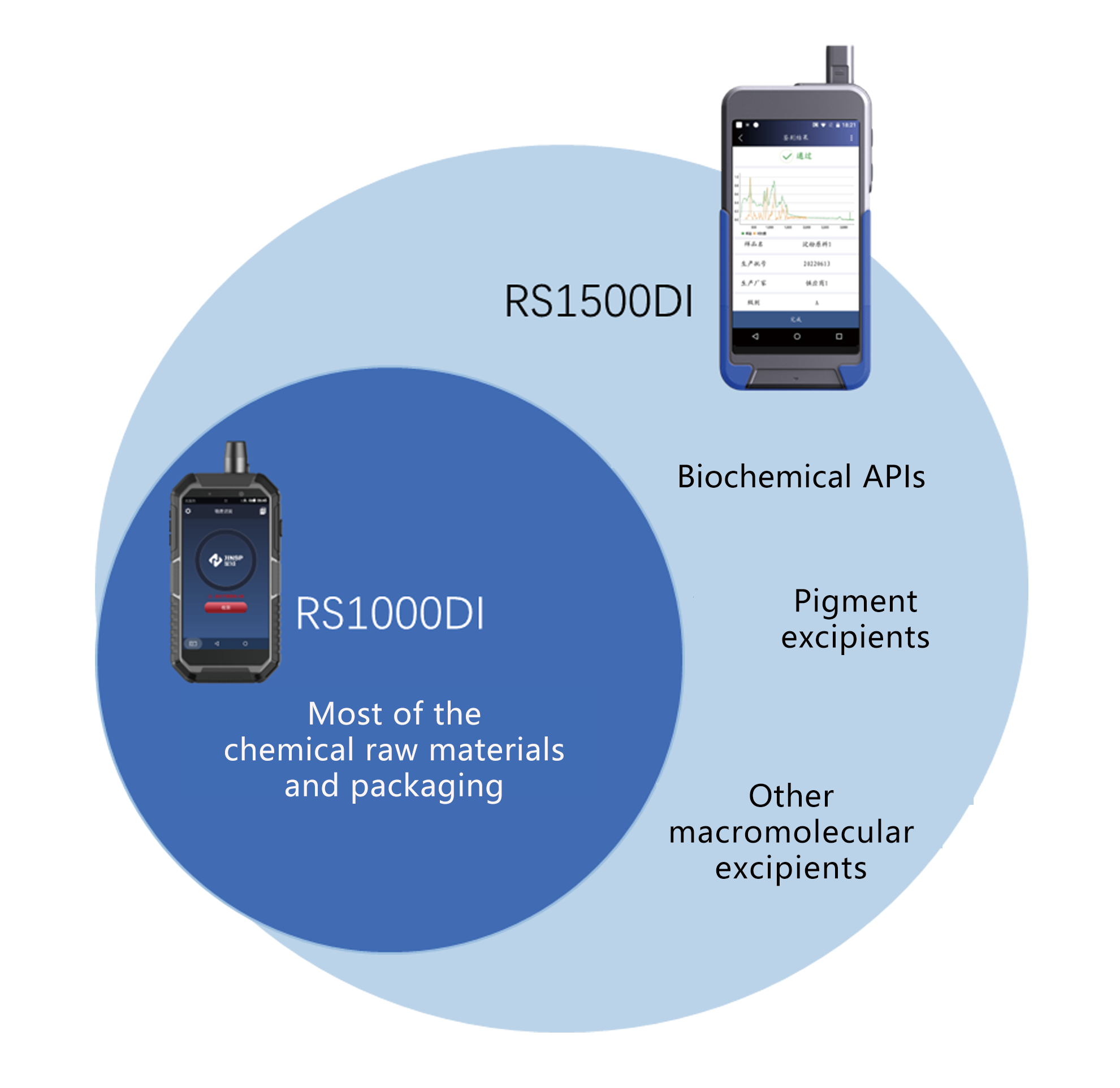
RS1000DI&RS1500DI
• Materiae rudis chemica: aspirin, acetaminophen, acidum folicum, niacinamide, etc.
• Pharmaceutical ex Galeno: salia, alkalis, saccharum, esters, alcohols, phenola, etc.
• materia packaging: polyethene, polypropylene, polycarbonata, ethylene-vinyl acetas copolymer
RS1500DI
• APIs biochemical: amino acida eorumque derivata, enzyma et coenzyma, proteins.
• Pigmentum ex Galeno: carmine, carotene, curcumino, chlorophyllo, etc.
• Exceptiones macromoleculares aliae: gelatina, cellulosa microcrystallina, etc.
RS1500DI:
| Specification | Descriptio |
| Technology | Raman Technology |
| Laser | 1064nm |
| Woctingenti | 730g (including altilium) |
| Connectivity | USB/Wi-FI/4G/ Bluetooth |
| Power | Rechargeable eu Li-ion altilium |
| Data format | SPC/txt/JEPG/ PDF |
RS1000DI:
| Specification | Descriptio |
| Laser | 785nm |
| Pondus | -500g (including altilium) |
| Connectivity | USB/Wi-FI/4G/ Bluetooth |
| Potestas | Rechargeable eu Li-ion altilium |
| Data forma | SPC/txt/JEPG/ PDF |
1. Cooperatio pharmaceutica internationalis Inspectio Programma (PIC/S) eiusque GMP Guidelines:
Appendix VIII Sampling of Rudis Materias et Packaging Materias The identificatio totius materiae massam confirmari potest solum postquam identificatio probata exercetur in speciminibus in unoquoque vase packaging.
2. US FDA praesens bonum faciens praxim US FDA GMP:
FDA 21 CFR Pars 11: Utraque pars pharmaci, saltem una identificatio, facienda erit;
FDA Inspectoris Instructio Manual: Actio saltem unum identitatis specificae test pro unaquaque massa cuiusque materiae rudis.